JAMP Pharma Corp., a Quebec pharmaceutical maker, is recalling one lot of JAMP-Pregabalin pain medicine because bottles labelled to contain 50-milligram capsules may contain 150-milligram capsules instead.
Health Canada announced the recall in an unusual Saturday evening statement.
The announcement said the company’s mix-up could lead to patients taking a much larger dose of the painkiller than prescribed, possibly resulting in an overdose that could “pose serious, potentially fatal health risks.”
JAMP-Pregabalin is an adult prescription drug. It is used to treat pain caused by nerve damage due to diabetes, shingles or spinal cord injury. It is also used to treat pain associated with fibromyalgia, Health Canada said.
The recall affects one lot of the 50-mg capsules bearing lot number 2305012747, which carries a 2026-08 expiry date, Health Canada said.
Health Canada stressed that taking too much pregabalin or suddenly increasing the dose could potentially lead to patients overdosing, which can be life-threatening.
The government urged both ordinary patients and health professionals, such as pharmacists, to check the contents of their JAMP-Pregabalin 50-mg bottles and see if they contain 150-mg capsules.
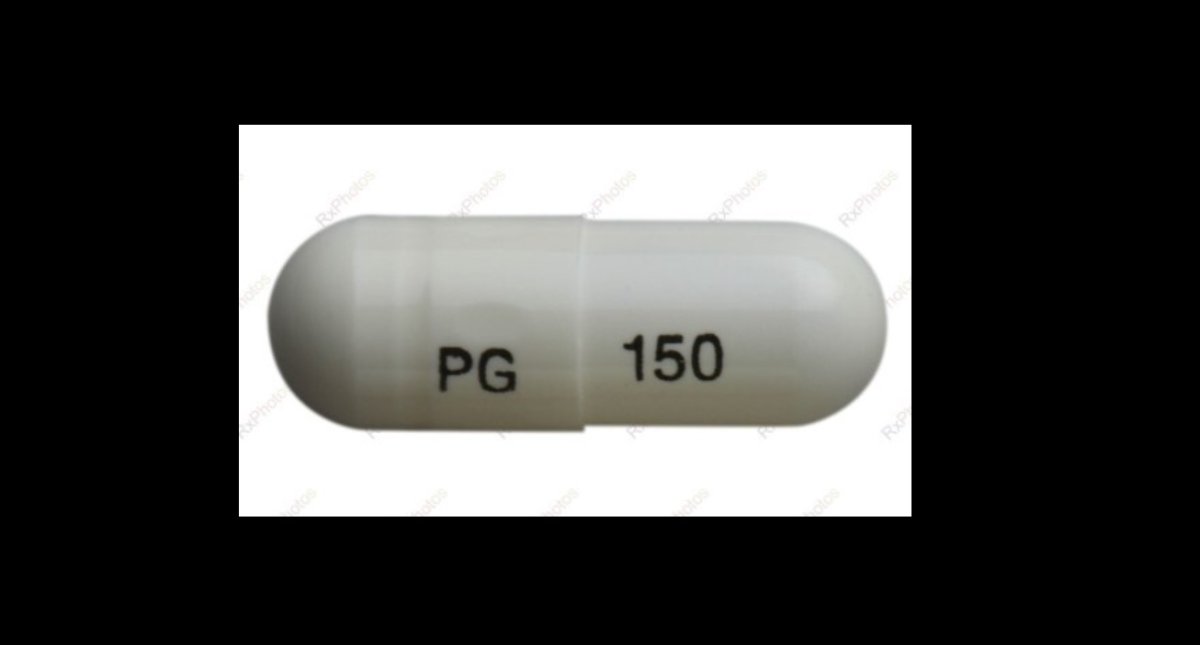
A 150-mg capsule of JAMP-Pregabalin pain medicine.
Health Canada
The agency warned that symptoms of pregabalin overdose may include sudden mood changes, sleepiness, confusion, depression, agitation, restlessness and seizures. It urged anyone taking the medication experiencing such symptoms to seek immediate medical attention.
It also stated that taking too much pregabalin while taking other drugs that act on the central nervous system, including opioids, “has been associated with heart electrical problems, seizures and death.”
Patients should not abruptly stop taking pregabalin, as this may result in withdrawal symptoms including insomnia, nausea, headache, anxiety, excessive sweating, diarrhea and convulsions, it said.
It is unclear how many bottles of medicine and how many capsules were included in the lot recalled or if anyone has been harmed.
Neither Health Canada nor JAMP Pharma immediately responded to questions about the recall.
Health Canada said it is monitoring JAMP Pharma’s recall and investigation of the mix-up, “including its implementation of corrective and preventive actions to prevent this issue from reoccurring.”
Patients are being advised to check their medication bottles or package very carefully.
“If your prescription is for 50 mg capsules and the bottle contains any 150 mg capsules, or if you are unsure, return it to your pharmacy immediately,” Health Canada said. “If you are unable to return your capsules to the pharmacy right away, talk to your pharmacist or doctor for further guidance.”